Biology: Chapter 4: Cell surface membrane: Movement in and out of cells
Five basic mechanisms by which exchange is achieved:
- Diffusion
- Faciliated diffusion
- Osmosis
- Active transport
- Bulk transport
Diffusion
- Net movement of particles from a region of high concentration to a region of lower concentration through random movement of it's molecules.
- Move down a concentration gradient
- Random movement caused by natural kinetic energy
- To reach equilibrium
Factors that affect diffusion rate across a membrane:
- Steepness of concentration gradient: The greater the difference in concentration, the faster the rate of diffusion.
More molecules will be moving from one side to another - Temperature: The higher the the temperature, the faster the rate of diffusion
Higher temperature means more kinetic energy - molecules move faster - Surface area: The greater the surface area, the faster the rate of diffusion
-The greater the surface area, the more molecules can cross it at any one moment.
-Surface area can be increased by folding
-The larger the cell, the smaller it's surface area is in relation to it's volume -volume increases more rapidly than surface area as size increases.
-Therefore there is a limit on the size of cells, since they rely on diffusion for internal transport.
-Time it takes for a molecule to reach it's destination by diffusion increases rapidly with distance traveled.
-Most cells are only 50 micrometers in diameter. - Nature of molecules or ions: Small, non-polar molecules diffuse faster through the membrane (eg. oxygen and carbon dioxide diffuse directly through phospholipid bi-layer).
-Non-polar molecules are not repelled by the hydrophobic interior of the phospholipid bi-layer.
-Large molecules require more energy to move, therefore diffuse more slowly.
-Water molecules, despite being polar, diffuse directly through the bi-layer because they are small enough to not be repelled by hydrophobic tails.
Facilitated diffusion
- Diffusion of large polar molecules and ions made possible by transport proteins.
- Two types of transport proteins: channel and carrier proteins.
- Each is highly specific and only lets one type of molecule/ion to pass through it.
Channel proteins
- Water-filled pores
- Fixed shape
- Allow charged substances usually ions, to diffuse through membrane.
- Usually 'gated' - Part of the protein molecule on the inside surface of the membrane can move to close or open the pore, like a gate.
- This allows control of ion exchange.
- eg. Nerve cell surface membrane channel proteins; one type allows entry of sodium ions for production of an action potential, while another allows the exit of potassium ions during recovery phase for repolarisation.
- Some channels occur in a single protein while others are formed by several proteins combined.
Carrier proteins
- Flip between two shapes
- The binding site alternately opens to one side of the membrane, then the other.
- The molecules will move down the concentration gradient across the membrane like in normal diffusion.
- Rate of diffusion depends on how many channel and carrier proteins the membrane has.
- In the case of carrier proteins, the rate of diffusion also depends on whether they are open or not.
- Cystic fibrosis is caused by a deficit in a channel protein that allows chloride ions to move out of the cells lining the lungs.
Osmosis
- Special type of diffusion involving only water molecules.
- Movement of water molecules by diffusion from a dilute solution to a concentrated solution through a partially permeable membrane.
- Remember: Solute + Solvent = Solution
- Two solutions separated by a partially permeable membrane - only allows certain molecules through.
- In solution A, there are more sugar molecules on the left than the right - the left is more concentrated.
- The partially permeable membrane only allows water molecules through.
- The water molecules diffuse through the membrane from the right to the left, down a concentration gradient due to random movements as to reach equilibrium
- In solution B, the left contains more water molecules, so it is more dilute, and the right contains less water, becoming more concentrated.
Water potential
- Tendency of water to move out of a solution.
- Depends on two factors:
-How much water the solution contains in relation to solutes (concentration)
-How much pressure is being applied - Water potential always moves down a water potential gradient (region of high water potential to a region to low water potential) until water potential is same throughout the system - equilibrium.
- A solution containing a lot of water (dilute) has a higher water potential than a solution containing a little water (concentrated).
- Increasing pressure on a solution also increases water potential - increases the tendency for water to move out of it.
- Water potential of pure water at atmospheric pressure is 0.
- Therefore a solution (water with solute/solutes dissolved in it) must have a negative water potential - less than 0.
Solute potential and pressure potential
- Solute potential: Extent to which solute molecules decrease the water potential of the solution.
- Solute potential is also 0 for pure water, and a negative value for a solution.
- Adding more solute to a solution decreases it's water potential.
- Pressure potential: Contribution of pressure to the water potential of a solution.
- Increasing pressure increases water potential.
Osmosis in animal cells
- If the water potential surrounding the cell is too high - cell swells and bursts (lysis)
- If it is too low - cell shrinks
- Essential to maintain a constant water potential in the bodies of animals
 Osmosis in a blood cell
Osmosis in a blood cell
- Hypotonic: Water potential surrounding cells too high
- Isotonic: Normal water potential surrounding cells
- Hypertonic: Water potential surrounding cells too low
Osmosis in plant cells
- Unlike animal cells, plants have a cell wall.
- Cell walls are freely permeable.
- When water potential is higher outside than inside the cell, water will enter the cell.
- However, as water enters plant cells the cell wall will push back against the expanding protoplast (living part of the cell) and pressure builds up.
- This is the pressure potential and it increases the water potential until the water potential inside and outside the cell are equal - equilibrium is reached.
- The cell wall is so inelastic that it takes very little water to enter the cell to achieve this.
- Cell wall prevents cell from bursting.
- A plant cell is fully turgid when it is fully inflated with water.
- Water potential = Solute potential + Pressure potential
 Osmosis in a plant cell
Osmosis in a plant cell
- Hypotonic: Fully turgid
- Isotonic: No net movement of water
- Hypertonic: Water leaves cell and protoplast gradually shrinks until it exerts no pressure on the cell wall - pressure potential 0.
As the protoplast continues shrinking it begins to pull away from the cell wall.
This is called plasmolysis.
When the protoplast has completely shrunken away from the cell wall, it is said to be fully plasmolysed. When the pressure potential has just reached 0 and plasmolysis is about to occur is called incipient plasmolysis.

Active transport
- Movement of molecules or ions through carrier proteins across a cell membrane against their concentration gradient using energy from ATP.
- ATP (produced during cell respiration) is used to make the carrier proteins change its shape, transferring molecules or ions across the membrane in the process.
- Can occur either into or out of the cell.
- Example: Sodium potassium (Na+ - K+) pump
- Found in cell surface membranes of all animal cells
- Run almost all the time, on average use 30% of a cells energy.
- For each ATP molecule used it pumps 3 sodium ions out of the cell while at the same time allowing 2 potassium ions into the cell.
- Net result is the inside of the cell becomes more negative than outside - potential difference (p.d) is created across membrane.
- The pump has a receptor for ATP on it's inner surface. The receptor acts as an ATPase enzyme in bringing about the hydrolysis of ATP to ADP (adenosine diphosphate) and phosphate to release energy.
 Sodium potassium pump
Sodium potassium pump
- Active transport is also important in re-absorption of ions and useful molecules in the kidneys after filtration and absorption of some products of digestion from the gut. In plants, active transport is used inorganic ions from soil to the root hairs and load sugars into the phloem tissues.
Bulk transport
- Bulk transport of large quantities of materials into (endocytosis) and out of (exocytosis) of cells.
- Endocytosis: Engulfing of a material by the cell surface membrane to form a small sac (endocytic vacuole). Two forms:
-Phagocytosis (cell eating): Bulk uptake of solid material. Cells specialising in this are called phagocytes, and the vacuoles are called phagocytic vacuoles. Example: White blood cells engulfing bacteria. Phagocytic vacuoles fuse with lysosomes, which contain digestive enzymes.
-Pinocytosis (cell drinking): Bulk uptake of liquid. Vacuoles formed are often really small, in which the process is called micropinocytosis.
- Protein receptors on the outer cell surface membrane detect the molecules that need to be transported and binds to them.
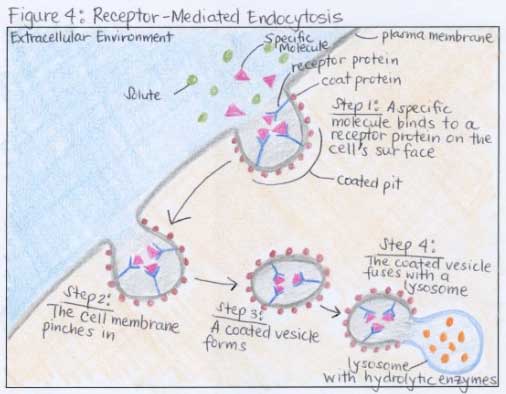 Endocytosis
Endocytosis
- Exocytosis: Reverse of endocytosis - materials are removed from cells. Usually involves golgi body. Example: In secretion of enzymes, secretory vesicles from the golgi body carry the enzymes to the cell surface to release their contents.
 Exocytosis
Exocytosis
 Simplified exocytosis and endocytosis
Simplified exocytosis and endocytosis
- Solute potential: Extent to which solute molecules decrease the water potential of the solution.
- Solute potential is also 0 for pure water, and a negative value for a solution.
- Adding more solute to a solution decreases it's water potential.
- Pressure potential: Contribution of pressure to the water potential of a solution.
- Increasing pressure increases water potential.
Osmosis in animal cells
- If the water potential surrounding the cell is too high - cell swells and bursts (lysis)
- If it is too low - cell shrinks
- Essential to maintain a constant water potential in the bodies of animals
 Osmosis in a blood cell
Osmosis in a blood cell- Hypotonic: Water potential surrounding cells too high
- Isotonic: Normal water potential surrounding cells
- Hypertonic: Water potential surrounding cells too low
Osmosis in plant cells
- Unlike animal cells, plants have a cell wall.
- Cell walls are freely permeable.
- When water potential is higher outside than inside the cell, water will enter the cell.
- However, as water enters plant cells the cell wall will push back against the expanding protoplast (living part of the cell) and pressure builds up.
- This is the pressure potential and it increases the water potential until the water potential inside and outside the cell are equal - equilibrium is reached.
- The cell wall is so inelastic that it takes very little water to enter the cell to achieve this.
- Cell wall prevents cell from bursting.
- A plant cell is fully turgid when it is fully inflated with water.
- Water potential = Solute potential + Pressure potential
 Osmosis in a plant cell
Osmosis in a plant cell- Hypotonic: Fully turgid
- Isotonic: No net movement of water
- Hypertonic: Water leaves cell and protoplast gradually shrinks until it exerts no pressure on the cell wall - pressure potential 0.
As the protoplast continues shrinking it begins to pull away from the cell wall.
This is called plasmolysis.
When the protoplast has completely shrunken away from the cell wall, it is said to be fully plasmolysed. When the pressure potential has just reached 0 and plasmolysis is about to occur is called incipient plasmolysis.
Active transport
- Movement of molecules or ions through carrier proteins across a cell membrane against their concentration gradient using energy from ATP.
- ATP (produced during cell respiration) is used to make the carrier proteins change its shape, transferring molecules or ions across the membrane in the process.
- Can occur either into or out of the cell.
- Example: Sodium potassium (Na+ - K+) pump
- Found in cell surface membranes of all animal cells
- Run almost all the time, on average use 30% of a cells energy.
- For each ATP molecule used it pumps 3 sodium ions out of the cell while at the same time allowing 2 potassium ions into the cell.
- Net result is the inside of the cell becomes more negative than outside - potential difference (p.d) is created across membrane.
- The pump has a receptor for ATP on it's inner surface. The receptor acts as an ATPase enzyme in bringing about the hydrolysis of ATP to ADP (adenosine diphosphate) and phosphate to release energy.
- Active transport is also important in re-absorption of ions and useful molecules in the kidneys after filtration and absorption of some products of digestion from the gut. In plants, active transport is used inorganic ions from soil to the root hairs and load sugars into the phloem tissues.
Bulk transport
- Bulk transport of large quantities of materials into (endocytosis) and out of (exocytosis) of cells.
- Endocytosis: Engulfing of a material by the cell surface membrane to form a small sac (endocytic vacuole). Two forms:
-Phagocytosis (cell eating): Bulk uptake of solid material. Cells specialising in this are called phagocytes, and the vacuoles are called phagocytic vacuoles. Example: White blood cells engulfing bacteria. Phagocytic vacuoles fuse with lysosomes, which contain digestive enzymes.
-Pinocytosis (cell drinking): Bulk uptake of liquid. Vacuoles formed are often really small, in which the process is called micropinocytosis. - Protein receptors on the outer cell surface membrane detect the molecules that need to be transported and binds to them.
 Endocytosis
Endocytosis- Exocytosis: Reverse of endocytosis - materials are removed from cells. Usually involves golgi body. Example: In secretion of enzymes, secretory vesicles from the golgi body carry the enzymes to the cell surface to release their contents.
 Exocytosis
Exocytosis
No comments:
Post a Comment